|
On Black Friday (in case you are wondering, the Friday
immediately following Thanksgiving was given the label
black because this first day of the holiday
shopping season is often very profitable for merchants
and traditionally
merchants when recording losses and profits in their
records would use red ink to indicate losses and black
ink to indicate profits) I went with my grandmother to
several electronics stores to pick out a new
television for a small desk in her kitchen area.
Because space is at a premium on her kitchen desk, I began
leaning toward flat panel displays as the best option for
her new television. However, as I began comparing
different models and prices, I realized that I really
had no idea how these extremely thin displays could
produce a picture without a cathode ray tube (CRT)
containing a scanning electron gun
exciting phosphor atoms on the screen. Plasma
and liquid crystal (LCD) displays accomplish
the task of producing a picture on a screen in very
different ways, but the ultimate product is a screen
only several inches thick. Let us begin with LCDs,
since they have been around longer, are more prevalent
in electronic devices other than televisions, and is
what I ultimately chose to get for my grandmother's
kitchen desk.
Phases of Matter
The fundamental element of a LCD that gives it its
name and ultimately
makes it work is the liquid crystal. At first,
this name seems to be a contradiction in terms. How
can something simultaneously have the properties of
both a liquid and a solid? We are all familiar with
the common states of matter: solids, liquids, and
gases. We might think of these states of matter as having
properties related to the characteristics of the
molecules which constitute a substance. That is, we
can characterize a substance as one of these states of
matter by looking at the properties of the molecules
which make up the substance. Solids are extremely ordered
substances, where molecules are arranged very neatly
in latticelike structures. In this state, the
molecules have definite position and orientation.
Liquids, on the other hand, have neither definite
position nor orientation. To elucidate this difference
between liquids and solids, consider a box of the
kid-favorite cereal rice krispies. In the box, these
rice krispies are free to shift and
rotate if the box is stirred or shaken. In the
cereal box, the rice krispies act much
like the molecules in a liquid. However, if you
take those rice krispies out of the box and mix them with a
melted marshmellow binder, you produce a rice krispie treat.
In this state, each rice krispie is locked into a
position and orientation by the marshmellow filler.
In a rice krispie treat, the rice krispies are much like
the molecules in a solid.

Microscopic View of a Liquid Crystal
Dr. Oleg Lavrentovich, Liquid Crystal Institute
What is a liquid crystal?
A liquid crystal is a phase of matter in between a solid and a
liquid. Similar to a solid, the molecules in a liquid
crystal tend to maintain their orientation, but
similar to a liquid, they are free to change their
position. At first, this seems quite odd. In a
solid, the orientation of the molecules is set by the
bonds which also establish their definite positions.
Therefore, if in a liquid crystal molecules are free
to change their positions, then the bonds between the
molecules cannot be the source of the orientations.
Instead, liquid crystals that have a definite order or
pattern (referred to as the nematic phase of liquid
crystals) have an orientation set by a
director. This director can be many different
things, including but not limited to an
electric/magnetic field or (and more relevant to LCDs)
a surface with microscopic grooves in it. It is
important to point out that on average the orientations
of the molecules of the liquid crystal are aligned
with the director of the crystal, such that not all
(and perhaps no molecules) will be perfectly aligned
with the director. Below is a diagram of a nematic
liquid crystal. Notice how only a few molecules are
perfectly aligned with the director,
labeled as the vector n, while most others are
nearly aligned with the director.
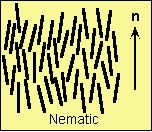
Diagram of a Nematic Liquid Crystal
Credit: Liquid Crystal Research Group at University
of Colorado.
Geometric Optics
Now that we have an idea of how liquid crystals are
structured, let us investigate how this physical
structure affects the optical properties of the
crystal (how the crystal interacts with light). In
general, light incident upon a barrier between two
different materials (such as glass and air) will
either be transmitted through the barrier
(refraction) or bounce
off of the barrier (reflection). The degree to
which the light is reflected or refracted at the
boundary is a function of what are called the
indices of refraction of the two materials
which meet at the boundary. The index of refraction
of material i, n, is given by
n = c/vi
where c is the speed of light in vacuum and
vi is the speed of light in material
i. Since the speed of light in vacuum is a
constant, a higher index of refraction implies a
slower speed of propagation for light waves in the
material.
|