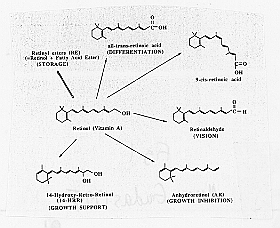
Many of the effects of retinoic acid on development occur through the ability of retinoic acid to regulate gene expression in various specific cell types. Retinoic acid binds to proteins in the steroid receptor family (Figure 1), and it is this RA receptor complex which mediates gene expression.

Figure 1: Structures of vitamin A and its derivatives (from Means and Gudas, 1995). Clockwise, beginning with retional (Vitamin A, in the center): Retinyl ester (RE) (STORAGE), all-trans-retinoic acid (DIFFERENTIATION), 0-cis retinoic acid, retinaldehyde (VISION), Anhydroretinol (AR) (GROWTH INHIBITION), 14-Hydroxy-Retro-Retinol (14-HRR) (GROWTH SUPPORT)
These proteins have been grouped into two subgroups, the retinoic response elements (RARs) and the retinoid "X" receptors (RXRs). The RARs a, b, and g bind to all-trans RA and its isomer 9-cis RA. The RXRs a, b, and g bind 9-cis RA, but not to all-trans RA with high affinity (Langston and Gudas, 1994). While most teratogenicity studies have focused on all trans-retinoic acid, its isomers 9-cis-retinoic acid and 13-cis-retinoic acid also may be somewhat teratogenic (Means and Gudas, 1995). Amino acid sequence comparisons have shown us that conservation of a given RAR or RXR type is greater between the species than is found between the three RAR or RXR types within a species. This conservation within a species has led to the conclusion that each RAR and RXR type has a unique function. They also have been found to synergize and activate several RA-responsive promoters (Chambon, 1993). One isoform of RAR a and one isoform of RAR g have been found to be essential for the transduction of the retinoid signal in embryos and adults. Mutation of these isoforms are lethal. Knocking out RAR a and RAR g in the limb bud, however, has not been found to effect limb morphogenesis, though its regulation is thought to be controlled by a morphogenetic gradient of RA (Chambon, 1993). Chambon (1993) mentions that isoforms which have been knocked out so far have no known phenotype. Mouse embryos which were null homozygous for RAR a and RAR g, though born without any abnormal phenotype, were either selectively eaten by the mother or died 1-2 weeks after birth. Their development was slowed and they became emaciated and lethargic, although no apparent malformations or lesion could be found (Chambon, 1993). Since growth continues after birth, the affects of these null mutations may not be affecting growth of limbs, nervous system, etc., when the embryo is initially developing, but it may be affecting the finishing touches of embryonic growth after birth when higher concentration levels may be required to regulate genes expressed or repressed at the end of embryonic development.
Another interesting finding is the high degree of functional redundancy between RAR isoforms. This is not expected because of the high conservation of all RARs and their isoforms and the specificity of their pattern of expression. The presence of duplications has likely occurred because the RARs came from a common ancestral gene, and thus have similar structures that allow it to perform similar functions. Each retinoic acid receptor is thought to have at least one unique function to "justify (its) striking conservation across vertebrates from fish to (hu)man" (Chambon, 1993).
The RAR and RXR genes have been found to activate or repress the expression of other genes. The RAR genes contain two promoters, which allow two different types of transcripts to be made. RARs can bind to RA response elements (RAREs) and directly activate gene expression Secondary changes in development not involving the binding of an RA-receptor complex to RARE, such as the expression of genes encoding for glycoproteins involved in the extracellular matrix, the tissue plasminogen activator, intermediate growth factors, can also occur through retinoic acid. While retinoic acid can regulate gene expression positively through the binding of the RA-receptor complex with RAREs, negative regulation is also mediated through an AP-1 binding site (Gudas, 1994). The RA-receptor complex prevents the activation of the genes by the AP-1 transcription factors. All cells examined so far have been found to express at least one of the RAR and RXR genes.
Retinoic acid itself is thought to be regulated by other proteins such as the cellular retinoic acid-binding proteins I and II (CRABPs I and II), which are cytoplasmic RA binding proteins thought to prevent retinoic acid from entering the nucleus by binding to it. Support for the regulation of retinoic acid function by CRABP proteins includes the finding that CRABP-I protein reduces the amount of RA available in the nucleus to regulate gene expression (Gudas, 1994). Because all cells with retinoic acid also have CRABP, the presence of CRABP is an indication that the cell requires retinoic acid for some aspect of development and cell differentiation (Maden et al., 1989). CRABPs may also direct the metabolism of retinoic acid into other important molecules with important biological functions. Maden et al. studied four stages of chick development from stage 16 to stage 24 while looking for the presence of CRABPs. CRABP antibody was stained in nine different cell populations:
The presence of CRABP cells in a wide variety of ectodermal and mesodermal cells demonstrate that retinoic acid may be expressed in the area as well. Maden et al. (1989) thus conclude that retinoic acid take up by the cells is concentrated in the neural crest region, an area in which retinoic acid was not thought to be present at the time. RA is likely being secreted from the dorsal blastopore lip. Organizer proteins emitted from the Nieuwkoop center in the dorsal blastopore lip may be indirectly affecting how much RA is being made by regulating retinoic acid expression. Maden et al. (1989) suggest that the teratogenicity of retinoids to the nervous system may occur because those tissues contain CRABP which may facilitate the uptake of retinoic acid into the cell and thus change normal gene expression.
Some of these genes that retinoic acid directly regulates are ones which function in both embryos and adults and encode other transcription factors. Other genes regulated by retinoic acid encode peptide growth factors (Means and Gudas, 1995). One of the key functions of retinoic acid is the regulation of homeobox genes. The homeobox genes are transcription factors which affect embryonic development. RA was initially associated with the regulation of Hox genes from studies examining the differentiation of teratocarcinoma cells. It was found that the responsiveness of a Hox gene to retinoic acid corresponds to its location in the gene cluster. Low levels of RA were found to induce the transcription of genes near the 3' end of the cluster, while genes farther away from the 3' end of the cluster required a longer time period and higher concentration of retinoic acid was required to activate the transcription of the Hox gene (Langston and Gudas, 1994). Although each gene in the cluster is being transcribed in the 5' to 3' direction, activation by retinoic acid occurs in the 3' to 5' direction in mammalian development. The expression patterns of Hox genes thus correspond linearly with the order of genes along the DNA.
Three models have been suggested to explain the mechanism in which RA regulates Hox gene expression (Figure 2). In the first model retinoic acid directly regulates Hox gene expression by binding RA to RAREs in the enhancer region of a Hox gene. RARE elements have been located in enhancers downstream from the murine Hox-a1 and human HoxA1 genes, and the murine and chicken Hox-b1 genes. RAR g is thought to activate the Hox-a1 gene in response to RA in the teratocarcinoma cells (Langston and Gudas, 1994). A second model supports the idea that one RARE coordinates the expression of several other Hox genes. The reduction in shading of the figure below depicts the notion that Hox gene activation by retinoic acid is gradient dependent. A higher concentration of retinoic acid is required to activate equivalently a gene near the 5' end than is required at the 3' end. The third model depicts the initiation of a cascade of gene activation of Hox proteins beginning with the activation of one 3' retinoic acid responsive enhancer. Each gene activates the adjoining gene on the 5' side and the Hox genes are thus activated sequentially (Langston and Gudas, 1994).

Figure 2: Models describing possible mechanisms for the activation of Hox gene clusters by retinoic acid. (from Langston and Gudas, 1994)
At the time the Langston and Gudas paper was published, it was still believed that a retinoic acid gradient may be controlling directly the expression of Hox genes, especially that of the Hox-d genes which are expressed around the zone of polarizing activities (ZPA) in the limb. It has since been determined that sonic hedgehog, a vertebrate homologue of the Drosophila segment polarity gene hedgehog , is the molecule made in the ZPA which coordinates the development of the anterior-posterior axis (Scott Gilbert, Personal Communication). Retinoic acid, however, coordinates its function with sonic hedgehog, and helps the functioning of the gene. The Bone Morphogenic Protein 2 (BMP2) also aids in the functioning of sonic hedgehog. Research is now being done to identify and elucidate the nature of the targets of the Hox genes.