Mechanical Influences of the Mesenchyme
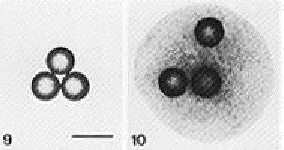
The epithelial layer of the developing submandibular gland becomes very plastic, which is unlike normal differentiated epithelium. Differentiated epithelium is traditionally characterized by having tight intracellular junctions and therefore being very rigid (Heida et. al., 1996). A study by Nogawa and Nakanishi (1987) showed that the flowing of mesenchymal cells was critical. Mesenchymal cells that are directly related to the branching morphogenesis of the epithelium have a better ability to move beads in culture than does mesenchyme that does not contact epithelium. The mesenchyme caused the separation of the beads by moving around in an aggregate in vitro as it has been shown to do in the salivary gland. It is this process of moving mesenchymal cells which is thought to act in cleft widening (Nogawa and Nakanishi, 1987) (Figure 1).

Figure 1: Box 9 shows three plastic balls not on mesenchyme. Figure 10 demonstrates the effect that mesenchyme has because the three balls cultured on mesenchyme were seperated by the flowing movement of the submandibular mesenchyme (Figure from Nogawa, 1987)
Collagen
Collagen may also play a role in causing the epithelium to branch into lobules. It is stimulated to be produced in the mesenchyme by the epithelium as the epithelium is making its basal lamina (Gilbert, 1994). There are multiple different types of collagens known to exist. Many are secreted into the basal lamina of the developing mouse submandibular gland by the underlying mesenchyme. An experiment by Fukada (1988) prompted the exploration of these various collagen types. This principal experiment showed collagen to play a large role in the initiation of cleft formation within the epithelium of the embryonic salivary gland. Each cleft contains a characteristic collagen type which begins to aggregate into bundles with the formation of the clefts (Fukada, 1988). This collagen is deposited into the pre-cleft areas by the mesenchymal cells, and their traction functions to align it into fibers. The clefts continue to get deeper as the mesenchymal cells continue to exert more traction (Figure 2). Collagen also provides a stratum for the flowing mesenchymal cells which produce a deforming force on the epithelial cells above them (Nakanishi and Sugiura, 1986).
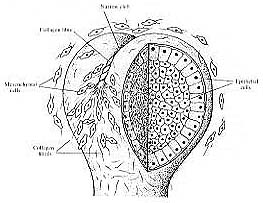
Figure 2: A possible model for cleft formation in the epithelium of mouse embryonic salivary glands. In the 12 or 13 embryonic day mouse collagen III begins to accumulate in fibers. These then contract and create traction which causes a cleft to form on the epithelial surface at the epithelial-mesenchymal interface. (Figure from Nakanishi et.al., 1986)
Nakanishi continued this research by examining the types of collagen present and their distributions. He found that it was most likely collagen III , which is located at every place the lower epithelial surface had a pre-cleft indent in a 12-day mouse. Later in this same 12-day stage the clefts became apparent and collagen III began to aggregate into bundles (Figure 3, Nakanishi, 1988). As branching of these glands progressed, the aggregates of Collagen III began to degrade showing that it doesn't play as large a role in the fully developed glands. No other collagen type present in the developing salivary gland, collagens I or IV had a distribution pattern which placed it in the developing clefts. Another collagen, type V collagen may have some importance as well, as it has been showed to help form collagen III bundles (Hardman, 1992). Collagen I is involved in maintaining connections between the mesenchymal layer and the epithelial layer though (Hardman, 1994) . Therefore, collagen I may have an important role in aiding the sliding mesenchyme deform the epithelium by causing junctions between the two layers.
In the presence of a collagenase inhibitor these cells also undergo more branching than normal (Nakanishi, 1986) (Figure 3).
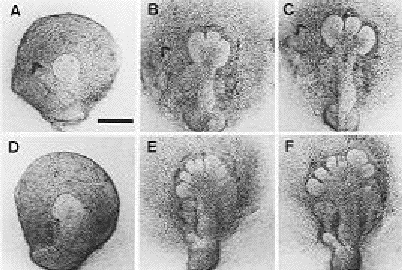
Figure 3: The effect of collagenase inhibitor on the branching of the mouse submandibular gland. There is significant more in the treated glands (bottom row) as compared to the branching of the control gland (top row) (Figure from Nakanishi, 1986).
The gland morphology seen in the presence of a collagenase is that of a globe; there is no branching as the collagen fibrils are broken up (Gilbert, 1994) (Figure 4). If the collagen fibrils are broken they cannot apply traction to the epithelium and cause cleft formation. These results further show that collagen may also a have a role in stabilizing and maintaining gland shape after morphogenesis.
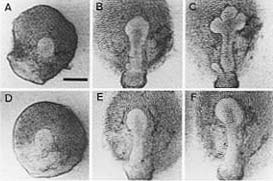
Figure 4: The effect of collagenase on the branching of the mouse submandibular gland. There is no branching in the treated gland as seen in the bottom row. The control gland in the top row exhibits normal branching (Figure from Nakanishi, 1986).
Link to mesenchyme